We discussed our novel cap analogs, CAP4 and CAP5, in the last blog The Evolution of Cap Analogs, you may wonder how we discovered these unique molecules. Areterna’s engine of innovation is built upon our AI-Assisted Cap Analog Discovery Platform which integrates a design-build-test-learn machine learning process, comprising five key steps.
Let’s delve into the intricacies of each step, unlocking the secrets of mRNA capping innovation.
Step 1: AI-Assisted Structure Design
At the nucleus of our platform is the AI-assisted structure design, a starting point for our cap analog discovery journey. Leveraging advanced algorithms, we initiate the process with a predefined structure (right). The AI then takes the reins, generating diverse in-silico variations. This step forms the bedrock of our approach, providing a multitude of potential candidates for further exploration.

Step 2: Molecular Docking Simulation
With a plethora of in-silico designs in hand, the next step is the molecular docking simulation. Here, the small molecules designed in the previous step are subjected to rigorous scrutiny. Through intricate simulations, these molecules are fitted with the eIF4E protein, unraveling insights into their binding strength and interaction dynamics. This crucial step serves as a filter, identifying molecules with the highest potential for success.

Step 3: Result Analysis
The wealth of data generated from the molecular docking simulations undergoes meticulous analysis in the third step. Molecules are ranked based on their binding strength to eIF4E. This ranking system becomes the compass guiding us toward the most promising candidates for further development.

Step 4: Candidate Molecule Synthesis
Armed with the knowledge of the top-ranked molecules, we transition to the synthesis phase. However, practicality is a key consideration. If the molecules prove challenging to synthesize, they are deemed of lower commercial value and are consequently dropped from the pool. On the contrary, easily synthesizable candidates move forward for performance testing, setting the stage for the ultimate assessment of their efficacy.
Step 5: Candidate Molecule Performance Testing
The culmination of our journey lies in the performance testing of selected molecules. These candidates undergo a series of evaluations, beginning with mRNA synthesis. The cap analogs will be used in IVT reactions and key IVT parameters such as yield, purity, capping efficiency, and dsRNA content are scrutinized. The table below shows data from 20ul IVT reactions where CAP4 and CAP5 demonstrated comparable performance to the benchmark m7GpppAmG.
| Cap Analog | mRNA Yield (μg) | mRNA Integrity | Capping Efficiency | dsRNA Ratio |
| m7GpppAmG | 183 | 90.20% | 98.50% | 0.04% |
| CAP 4 | 187 | 91.30% | 99.10% | 0.03% |
| CAP 5 | 184 | 89.50% | 98.90% | 0.04% |
Molecules demonstrating robust performance are further tested for their resistance to decapping enzymes. The table below shows the percentage of mRNA with a cap on after one hour of incubation with decapping enzymes. CAP4 and CAP5 outperformed the benchmark m7GpppAmG.
| Cap Analog | Remaining Cap% -1 | Remaining Cap% – 2 | Remaining Cap% -3 |
| m7GpppAmG | 22.50% | 19.30% | 28.40% |
| CAP 4 | 77.30% | 82.50% | 84.00% |
| CAP 5 | 35.90% | 35.20% | 34.40% |
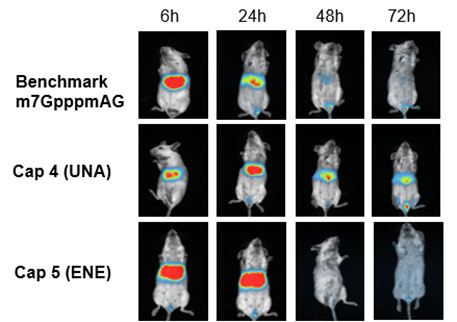
The final candidates are tested for protein expression in cells using GFP construct. Finally, luciferase mRNAs are made with cap analogs and injected into mice to assess protein expression in vivo.
The mRNA capped with m7GpppAmG showed strong luciferase signal at the 6-hour time point, but the luciferase signal was almost all gone at 48 hours. In contrast, CAP4 mRNA showed weaker expression at 6 hours, but the protein expression impressively persisted beyond 72 hours. CAP5 mRNA gave the highest protein expression initially among the 3 constructs and the strong luciferase signal carried from 6 hours to 24 hours, then died down at 48 hours.
Based on the performance data, we launched CAP4 and CAP5 as commercial products.
These and other performance data with related molecular structures will be fed into the AI system to train the model for future development.
In essence, our AI-assisted cap analog discovery platform provides an engine of innovation. We will not stop at CAP4 and CAP5, more novel cap analogs will come out of the pipeline in the future.