Stay ahead in mRNA research and development with our newly available capped and uncapped reference standards!
A linear mRNA molecule primarily consists of five components: the 5′ cap structure (5′ Cap), the 5′ untranslated region (5′ UTR), the open reading frame encoding the protein, the 3′ untranslated region (3′ UTR), and the PolyA tail. Currently, the most efficient method for large-scale mRNA production is in vitro transcription (IVT). The complexity of mRNA structure and its production process make the analysis of mRNA drug products more challenging than that of traditional biological products. However, precise analysis of various indicators of mRNA products can evaluate the efficacy and safety of mRNA. Therefore, the development and validation of analytical methods are crucial.

Key Quality Attribute of mRNA: Capping Efficiency
The cap structure plays an important role in mRNA function, facilitating translation initiation by binding to eukaryotic initiation factor (eIF4E) and protecting mRNA from degradation. It also enhances stability by promoting mRNA circularization. The development and validation of accurate capping efficiency methods are important to understanding the mRNA’s in vivo performance.
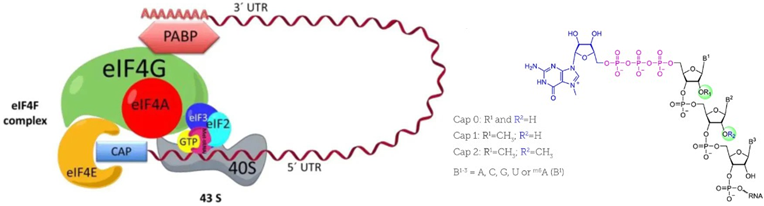
How RNase H is Used to Determine Capping Efficiency
Due to the large molecular weight of full-length mRNA, mass spectrometry cannot capture the entire signal of the larger fragments. As a result, the commonly used method for determining capping efficiency during production involves RNase H-mediated probe cleavage. This method utilizes biotin-labeled probes, which are specifically captured by streptavidin-coated magnetic beads. The 5′ end sequence of the mRNA binds to the probe, enabling RNase H to recognize and cleave the mRNA. After washing and elution, the 5′ single-stranded oligonucleotide sequence is obtained. LC-MS is then used to analyze the molecular weight and structure of the mRNA fragments, providing a quantitative evaluation of the capping efficiency.

Figure 3 Schematic diagram of the principle of H-mediated probe cleavage[3]
Our Capped and Uncapped Reference Standards
Our reference standards are designed for use in capping efficiency testing, and they are mixed in the correct molar ratios to validate linearity and accuracy. The results of our validation show that these standards deliver excellent performance across multiple levels of accuracy.
- Capped Standard: Positive control. Contains a high percentage of capped mRNA molecules, confirming the assay’s ability to detect capped species.
- Uncapped Standard: Negative Control. Contains a very low percentage of capped mRNA, establishing a baseline and validating the assay’s specificity.
Conclusion
The 5′ cap structure is essential for mRNA stability and efficient protein translation. High capping efficiency optimizes both immunogenicity and translation efficiency, making it a critical quality attribute for mRNA vaccines and drugs. As a leading supplier of mRNA raw materials, we provide not only cap analog raw materials with no expensive licensing fees, but also customized reference standards and capping efficiency testing services to meet the needs of diverse mRNA product developments. By incorporating these standards into your workflow, you can confidently validate your analytical methods, troubleshoot production issues, and ensure the quality of your mRNA products. The results of our validation studies demonstrate their excellent performance across multiple levels of accuracy.
Our One-Stop Solution for mRNA Capping:
- Extensive cap analog product range for various mRNA needs
- Independent intellectual property rights for cap analogs to mitigate patent issues
- Comprehensive mRNA capping efficiency testing services
- Custom reference standards for method validation to support IND applications
Sources:
- Namit Chaudhary, Drew Weissman, Kathryn A. Whitehead. “mRNA vaccines for infectious diseases: principles, delivery, and clinical translation“, Nat Rev Drug Discov. 2021.
- Montero H, R García, Mora SI. “eIF4E as a Control Target for Viruses”, Viruses, 2015.
- Beverly M, Dell A, Parmar P, et al. “Label-free analysis of mRNA capping efficiency using RNase H probes and LC-MS“, Anal Bioanal Chem. 2016.
