We have discussed the evolution of cap analogs and the new generation of fine-tuned cap analogs for different applications. Generally, there are two ways of capping mRNA produced from an IVT reaction. One way is co-transcriptionally capping where a cap analog is added to the IVT reaction and gets incorporated into the 5’- terminal of the mRNA by the T7 RNA Polymerase. Another method is enzymatic capping post IVT.
Enzymatic Capping Process
The enzymatic capping process involves two enzymes and a series of reactions. The RNA generated by IVT needs to be purified first to get rid of NTPs, DNA, and enzymes. Then 4 ingredients are added to make mRNA – GTP, Vaccinia capping enzyme, 2´-O-methyltransferase, and S-adenosyl-methionine (SAM or AdoMet). The enzymatic reaction takes 4 steps as outlined below –
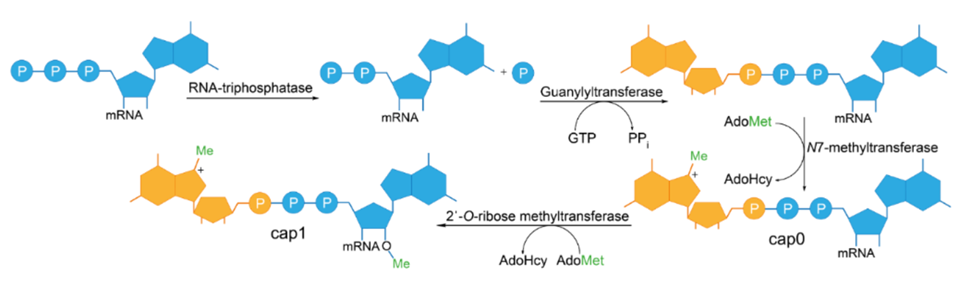
1) RNA-triphosphatase removes the γ-phosphate from the 5’-ppp RNA.
2) Guanyltransferase couples a GMP to the resulting 5’-pp RNA.
3) N7-methyltransferase transfers a methyl group from SAM to the terminal guanine to generate Cap0 structure.
4) 2-O-ribose methyltransferase methylates the 2’OH of the penultimate guanine to produce Cap 1.
The first 3 steps are carried out by the Vaccinia virus Capping Enzyme (VCE), which comprises two subunits, D1 and D12. The D1 subunit carries all three enzymatic activities—RNA triphosphatase, guanylyltransferase, and guanine methyltransferase. The triphosphatase and guanylyltransferase activities are located in the N-terminal half, while the methyltransferase is in the C-terminal half of the large D1 protein. The smaller D12 protein, although lacking catalytic activity, plays a crucial role in activating D1.
The enzymatic capping takes about one hour at 37°C. When optimized, the process is nearly 100% efficient, ensuring that all capped structures are added in the proper orientation.
Pros and Cons
The advantage of enzymatic capping is its high capping efficiency, and the manufacturing process is accessible to all. However, this method requires additional purification and enzymatic reaction steps which lead to the following downside –
- Longer manufacturing time
- Higher cost due to additional units of operation
- Higher QC and CMC burden
- Product loss from additional purification steps
In addition, the enzymatic steps may not run at 100% efficiency, for example, S-adenosyl-L-methionine (SAM) is prone to degradation and may affect the last two steps of the process. Some mRNA constructs may have secondary structures that affect the accessibility of the 5’-end to the capping enzyme.
Enzymatic capping certainly offers an advantage over ARCA co-capping which suffers from low capping efficiency and Cap0 limitation. However, m7GpppAmG and its derivatives can produce >95% capping efficiency and a simpler manufacturing process. Co-capping with Cap4 (UNA) or Cap5 (ENE) should be the process of choice for the future.
Reference –
- Muttach, F.; Muthmann, N.; Rentmeister, Synthetic mRNA capping. A. Beilstein J. Org. Chem. 2017, 13, 2819–2832. doi:10.3762/bjoc.13.274
- Norbert Pardi, Michael J Hogan, Drew Weissman, Recent advances in mRNA vaccine technology, Current Opinion in Immunology, Volume 65, 2020, Pages 14-20; https://doi.org/10.1016/j.coi.2020.01.008
