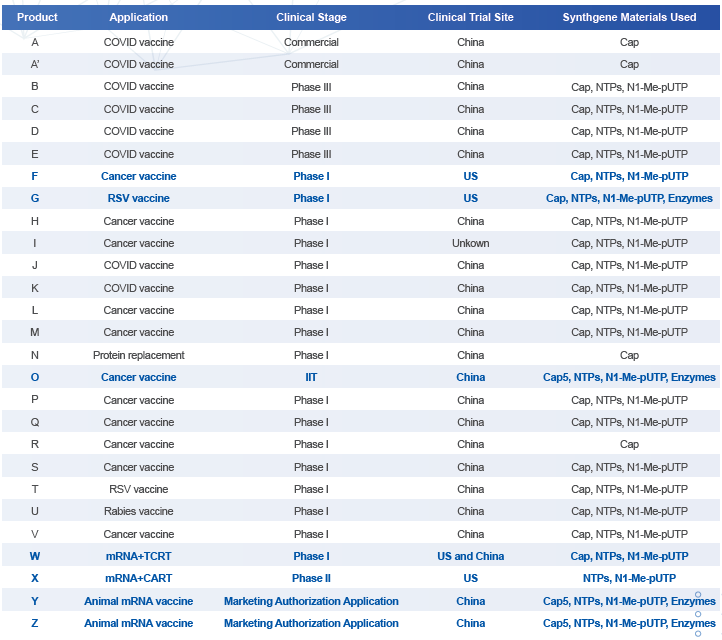
Synthgene Biotechnology, Areterna’s parent company, has been a leading producer of GMP-grade cap analogs, NTPs, and modified NTPs for several years, establishing a strong reputation for quality and reliability in the industry. Our raw materials play a crucial role in over 25 clinical mRNA programs, addressing a diverse range of medical needs. These programs include the development of COVID-19 vaccines, which have been critical in the global fight against the pandemic, as well as innovative cancer vaccines that offer new hope for oncology treatments. Additionally, our products are integral to advanced therapies such as T-cell therapy, which harnesses the body’s immune system to target diseases, and protein replacement therapies, which provide essential proteins that patients may lack due to genetic conditions.
To further support our clients and their clinical trials in the United States, we have secured a Drug Master File (DMF) with the FDA for selected raw materials. This DMF filing ensures that our products meet stringent regulatory standards, facilitating a smoother and more efficient approval process for our customers’ therapeutic products. By providing high-quality raw materials and regulatory support, Synthgene is committed to advancing the development and success of mRNA-based therapies and other cutting-edge medical treatments.
Clinical Trials Using Synthgene’s GMP Products
Note: For confidentiality, letters have been used to represent each clinical trial.
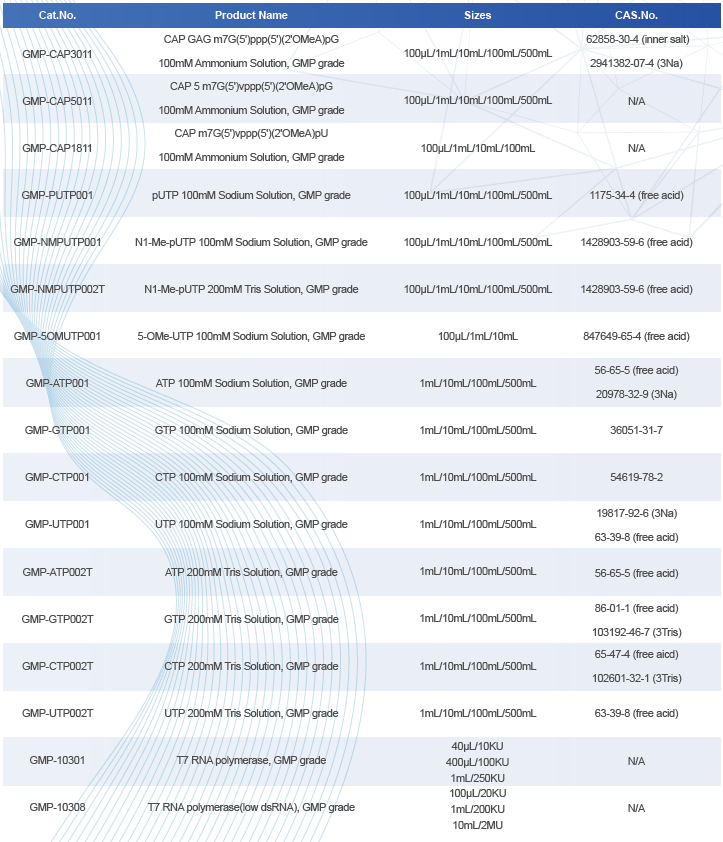
Areterna’s GMP Products
For inquiries about our GMP products, please email us at sales@areterna.com or visit our GMP Grade Products page to complete the form.
