Introduction
Modified nucleoside triphosphates (NTPs) are the master keys that unlock the therapeutic potential of mRNA. By strategically incorporating these chemically modified building blocks during mRNA synthesis, researchers can enhance stability, translation efficiency, and immunogenicity. The ability to have precise control over mRNA paves the way for their widespread application in vaccines, protein replacement therapies, and beyond.
The Impact of Modified NTPs
Traditionally, unmodified mRNA is rapidly degraded by cellular enzymes and triggers unwanted immune responses, limiting its therapeutic potential. Modified NTPs address these challenges by:
- Increased Stability: Modifications such as pseudouridine (Ψ) and N1-methylpseudouridine (m1Ψ) enhance mRNA stability by conferring resistance to degradation by ribonucleases. This improved stability leads to prolonged protein expression, crucial for therapeutic efficacy [1].
- Reduced Immunogenicity: Incorporation of modified NTPs like Ψ and m1Ψ can reduce the activation of innate immune sensors, such as Toll-like receptors (TLRs), which recognize foreign RNA. This dampened immune response minimizes inflammation and improves the safety profile of mRNA therapeutics [1].
- Enhanced Translation: Certain modified NTPs can enhance the translation efficiency of mRNA, leading to increased protein production. This is achieved by optimizing codon usage and improving ribosome binding and processivity [1].
N1-methylpseudouridine (m1Ψ): A Key Player in COVID-19 Vaccines
The significance of modified NTPs is exemplified by the use of m1Ψ in the Pfizer/BioNTech and Moderna COVID-19 vaccines. Replacing uridine with m1Ψ in the mRNA sequence encoding the SARS-CoV-2 spike protein significantly increased mRNA stability and translation, resulting in robust antibody production and protective immunity [2][3].
Introducing Areterna’s NMPUTP
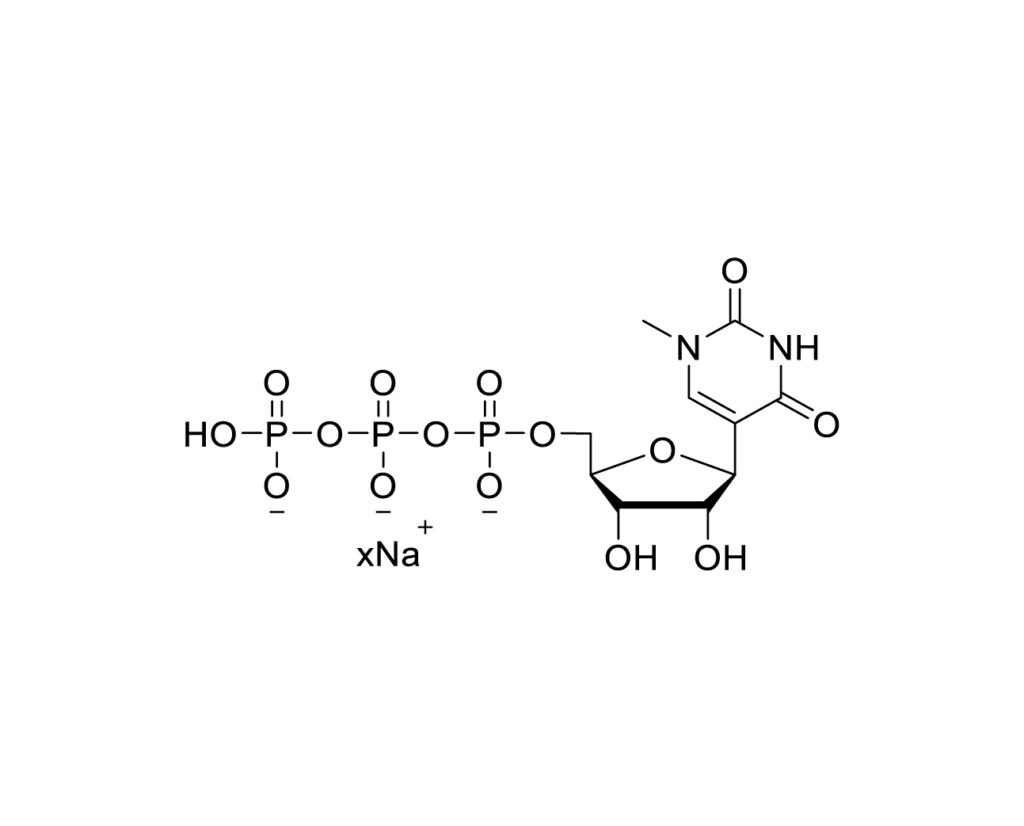
Areterna’s NMPUTP (N-methyl-pseudouridine-5′-triphosphate) has been proven to provide enhanced stability, translation efficiency, and safety in versatile applications of mRNA therapeutics.
Featured Product:
Name: N1-Me-pUTP 100mM Sodium Solution
Catalog Number: NMPUTP001
Sizes Available: 100μL;1mL;10mL;100mL;500mL
Purity: >99%
Here are a few examples of our NMPUTP001 being referenced in published research studies:
- Herpes Zoster Vaccine: In a study published in Emerging Microbes & Infections, Areterna’s NMPUTP played a crucial role in the development of a herpes zoster mRNA vaccine demonstrating superior immunogenicity [4]. This highlights NMPUTP’s ability to enhance the efficacy of prophylactic mRNA vaccines.
- Hepatitis B Vaccine: A study featured in npj Vaccines demonstrated the successful application of our NMPUTP in a therapeutic hepatitis B mRNA vaccine [5]. This vaccine exhibited strong immunogenicity and persistent virological suppression, showcasing the potential of NMPUTP to revolutionize the treatment of chronic viral infections.
- Rabies Vaccine: Research published in Frontiers showcased the power of our NMPUTP in a nucleoside-modified rabies mRNA vaccine[6]. The incorporation of NMPUTP contributed to the induction of prolonged and highly protective immune responses in mice after a single vaccination, underscoring its potential for developing effective and durable vaccines against infectious diseases.
- Gene Editing: Research published by Cell demonstrated its utility in all-RNA-mediated targeted gene integration using rationally engineered R2 retrotransposons[7].
Conclusion
Modified NTPs have revolutionized the field of mRNA therapeutics by overcoming the limitations of unmodified mRNA. Areterna is at the forefront of developing novel modified NTPs for mRNA therapeutics, with its extensive library of over 150+ modified NTPs, custom capabilities, and a commitment to ongoing innovation. As research continues, we can expect further innovations in modified NTPs, driving the development of next-generation mRNA therapeutics for a wide range of diseases.
Sources and related content
[1] Liu, A., & Wang, X. (2022). The Pivotal Role of Chemical Modifications in mRNA Therapeutics. Frontiers in Cell and Developmental Biology, 10, 901510.
[2] Nance, K. D., & Meier, J. L. (2021). Modifications in an Emergency: The Role of N1-Methylpseudouridine in COVID-19 Vaccines. ACS Central Science, 7(5), 748-756.
[3] Morais, P.; Adachi, H.; Yu, Y-T. (2021). The Critical Contribution of Pseudouridine to mRNA COVID-19 Vaccines. Frontiers in Cell and Developmental Biology, 9, 789427.
[4] Huang, L., Zhao, T., Zhao, W., Shao, A., Zhao, H., Ma, W., Gong, Y., Zeng, X., Weng, C., Bu, L., et al. (2024). Herpes zoster mRNA vaccine induces superior vaccine immunity over licensed vaccine in mice and rhesus macaques. Emerging Microbes & Infections, 13(1), 2309985.
[5] Zhao, H., Shao, X., Yu, Y., Huang, L., Amor, N. P., Guo, K., Weng, C., Zhao, W., Yang, A., Hu, J., et al. (2024). A therapeutic hepatitis B mRNA vaccine with strong immunogenicity and persistent virological suppression. npj Vaccines, 9(1), 22.
[6] Bai, S., Yang, T., Zhu, C., Feng, M., Zhang, L., Zhang, Z., et al. (2023). A single vaccination of nucleoside-modified rabies mRNA vaccine induces prolonged highly protective immune responses in mice. Frontiers in Immunology, 13, 1099991.
[7] Chen, Y., Luo, S., Hu, Y., Mao, B., Wang, X., Lu, Z., Shan, Q., Zhang, J., Wang, S., Feng, G., et al. (2024). All-RNA-mediated targeted gene integration in mammalian cells with rationally engineered R2 retrotransposons. Cell, 187, 4674-4689.
